- Home
- Application
- Chromatography
- Introduction to Chromatography
Introduction to Chromatography
| -What is Chromatography? -History and Development -Basic Principles (Adsorption, Partitioning, etc.) -Components of a Chromatographic System |
What is Chromatography?
An Essential Separation Science for Analytical and Preparative Applications
Introduction
Chromatography is a powerful and widely used analytical technique that separates, identifies, and quantifies components in complex mixtures It plays a critical role in scientific research, quality control, pharmaceutical development, environmental analysis, food safety, biotechnology, and countless industrial processes.
Definition
Chromatography is a physicochemical separation method in which the components of a mixture are distributed between two phases:
.Mobile phase: a fluid that moves through the system (liquid or gas)
.Stationary phase: a solid or liquid supported on a solid, which remains fixed inside a column or on a flat surface
The different components in a sample travel at different rates due to variations in their interactions with the stationary and mobile phases, leading to their separation.
Why is Chromatography Important?
Chromatography is essential in both qualitative and quantitative analysis, offering:
.High-resolution separation of complex mixtures
.Precise identification of compounds (even at trace levels)
.Purification of specific substances for further use
.Compatibility with a wide range of detectors (e.g., UV, MS, FID)
Applications span across:
Pharmaceuticals: drug analysis, impurity profiling, stability testing
Food Industry: detection of additives, pesticides, and contaminants
Environmental Monitoring: analysis of pollutants in air, water, soil
Clinical Diagnostics: biomarker and metabolite detection
Forensics: toxicology, doping control, and criminal investigations
Fundamental Principle
The underlying principle of chromatography is differential partitioning between phases. Each analyte in a sample interacts uniquely with the stationary and mobile phases based on properties such as:
.Polarity
.Molecular size
.Affinity to functional groups
.Solubility
These differences cause the analytes to migrate through the system at different speeds, resulting in a chromatogram — a graphical representation of detector response vs. time.
Basic Components of a Chromatographic System
1.Mobile Phase: Gas (e.g., helium) or liquid (e.g., methanol-water mix) that carries the sample
2.Stationary Phase: Column material or surface where separation occurs
3.Injector: Introduces the sample into the system
4.Column: Contains the stationary phase
5.Detector: Senses and records the separated components
6.Data System: Converts detector signals into readable chromatograms
Types of Chromatography
Chromatography can be classified based on physical state, mechanism of separation, or equipment design. Major types include:
1. Liquid Chromatography (LC)
.HPLC: High-Performance Liquid Chromatography
.UHPLC: Ultra-High-Pressure Liquid Chromatography
.Used for thermally unstable or high-molecular-weight compounds.
2. Gas Chromatography (GC)
.Best for volatile and thermally stable analytes
.Often coupled with Mass Spectrometry (GC-MS)
3. Thin Layer Chromatography (TLC)
.Fast, inexpensive technique using coated glass/plastic plates
.Widely used for screening, purity checks
4. Paper Chromatography
.Simpler variant of TLC, often used in educational settings
5. Ion Exchange Chromatography
.Separates ions and polar molecules based on charge
6. Size Exclusion Chromatography (SEC)
.Also known as Gel Filtration; separates by molecular size
7. Affinity Chromatography
.Highly selective; used to purify biomolecules using specific binding interactions
Key Advantages of Chromatography
.High accuracy and sensitivity
.Applicable to a wide variety of sample types
.Scalable from microgram to kilogram quantities
.Compatibility with automation and hyphenated techniques (e.g., LC-MS, GC-MS)
Limitations
.Can require complex sample preparation
.Instrumentation and columns can be costly
.Some methods require skilled operation and interpretation
Modern Trends in Chromatography
.Green Chromatography: eco-friendly solvents, reduced waste
.Miniaturization: microfluidic and portable devices
.Hyphenated Techniques: LC-MS, GC-MS, LC-NMR, expanding capabilities
.AI-Driven Data Processing: automated peak recognition and prediction
.Regulatory Compliance: growing role in validated methods (e.g., ICH, USP)
History and Development of Chromatography
From Colorful Origins to Cutting-Edge Analytical Science
Introduction
The journey of chromatography spans over a century, evolving from a simple color-based separation method into a cornerstone of modern analytical chemistry. Its history reflects the ever-growing demand for more precise, faster, and more efficient techniques to separate and analyze complex mixtures in various scientific and industrial fields.
Origins: The Birth of Chromatography (Early 1900s)
The term “chromatography” comes from the Greek words “chroma” (color) and “graphein” (to write). The technique was first introduced by the Russian botanist Mikhail Tswett in 1903, who used it to separate plant pigments like chlorophyll and carotenoids.
Key Contribution by Tswett (1903):
.Used calcium carbonate as the stationary phase and petroleum ether as the mobile phase
.Demonstrated that different pigments could be separated based on their movement through the column
.Named the method “chromatography” due to the colorful bands observed
Although initially overlooked, Tswett’s work laid the foundation for a revolution in separation science.
Mid-20th Century: Foundation of Modern Chromatography
1940s – Partition Chromatography
.Archer J.P. Martin and Richard L.M. Synge developed partition chromatography, where two liquid phases were used for separation.
.Their work led to the Nobel Prize in Chemistry (1952).
.This innovation greatly improved the ability to analyze amino acids and small organic molecules.
1950s – Paper & Ion Exchange Chromatography
.Paper Chromatography became popular for biochemical and educational applications.
.Ion Exchange Chromatography emerged for the separation of charged molecules, including proteins and nucleotides.
1952 – Gas Chromatography (GC)
.Anthony T. James and Archer J.P. Martin introduced gas-liquid chromatography, enabling high-resolution analysis of volatile compounds.
.GC became crucial in petrochemicals, flavors, and forensic toxicology.
1960s–1980s: Emergence of High-Performance Techniques
Thin Layer Chromatography (TLC)
.Developed as a faster alternative to paper chromatography
.Used solid adsorbent layers (e.g., silica) coated on glass or plastic
.Quickly adopted for purity checks and screening
High-Performance Liquid Chromatography (HPLC)
Emerged in the late 1960s to early 1970s as a response to limitations in traditional column chromatography
Key innovations:
.Use of high-pressure pumps for faster flow rates
.Smaller particle sizes for improved resolution
.Automated sample injection and data processing
Size Exclusion and Affinity Chromatography
.Introduced for the biological and pharmaceutical sectors
.Enabled protein purification, antibody isolation, and enzyme kinetics
1990s–2000s: Hyphenation and Integration
LC-MS and GC-MS
Coupling of chromatography with mass spectrometry transformed chemical analysis
Applications exploded in:
.Pharmaceuticals (drug development and pharmacokinetics)
.Environmental monitoring
.Clinical and forensic testing
Automation and Software
Full integration of computerized data systems (CDS)
Introduction of user-friendly platforms like:
.Empower (Waters)
.Chromeleon (Thermo)
.OpenLab (Agilent)
2010s–Present: Advanced Instrumentation and Green Technologies
Ultra-High-Performance Liquid Chromatography (UHPLC)
.Improved pressure tolerance (>15,000 psi)
.Faster run times with sharper peaks
.Reduced solvent consumption and sample volume
Micro and Nano-Chromatography
.Used in proteomics and metabolomics
.Ideal for working with extremely small sample volumes
Green Chromatography
.Emphasis on eco-friendly solvents, energy efficiency, and reduced waste
.Development of bio-based stationary phases and supercritical fluid chromatography (SFC)
Key Milestones Timeline
| Year | Development |
| 1903 | Tswett invents chromatography |
| 1940 | Partition chromatography developed |
| 1952 | Gas chromatography introduced |
| 1960 | Ion exchange and paper chromatography popularized |
| 1970 | HPLC emerges as a standard |
| 1990 | Rise of LC-MS and GC-MS |
| 2000 | Automation and digital control systems |
| 2010 | UHPLC and green technologies |
| 2020 | AI-driven chromatography, remote monitoring, lab-on-chip |
Basic Principles (Adsorption, Partitioning, etc.)
Chromatography is a powerful separation technique that operates on the fundamental principle of differential distribution of components between two phases: a mobile phase (gas or liquid) and a stationary phase (solid or liquid). The interactions between analytes and these phases determine the speed at which they travel through the chromatographic system, enabling their separation and identification.
Adsorption Chromatography
Adsorption chromatography relies on the surface interaction between analyte molecules and a solid stationary phase, such as silica or alumina. Molecules with stronger adsorption forces move slower through the system.
.Commonly used in Thin Layer Chromatography (TLC) and Column Chromatography
.Suitable for separating non-volatile organic compounds
.Retention depends on polarity, surface area, and solvent strength
Key Factors:
.Surface characteristics of the adsorbent
.Polarity of the analytes
.Eluting solvent composition
Partition Chromatography
In this mode, separation occurs through the differential solubility of compounds in two immiscible phases: a liquid stationary phase bonded to a solid support and a liquid or gas mobile phase.
.Used in High-Performance Liquid Chromatography (HPLC) and Gas-Liquid Chromatography (GLC)
.Especially effective for neutral and polar organic compounds
Key Concepts:
.Partition coefficient (K)
.Polarity of mobile and stationary phases
.Temperature and flow rate
Ion Exchange Chromatography (IEC)
Ion exchange chromatography separates molecules based on electrostatic interactions between charged analytes and an oppositely charged ion exchange resin.
.Used for amino acids, proteins, nucleotides, and inorganic ions
.Stationary phases are typically functionalized polymers with sulfonic (cation exchanger) or quaternary amine groups (anion exchanger)
Key Parameters:
.pH and ionic strength of the mobile phase
.Type and capacity of resin
.Elution gradient (salt or pH)
Size Exclusion Chromatography (SEC)
Also called Gel Filtration Chromatography, this technique separates molecules based on molecular size. The stationary phase contains porous beads that allow smaller molecules to enter, causing larger molecules to elute first.
.Used for proteins, polymers, and macromolecules
.No interaction with stationary phase—separation is purely physical
Advantages:
.Gentle on biological samples
.Provides molecular weight estimation
.High reproducibility and resolution for size-based separations
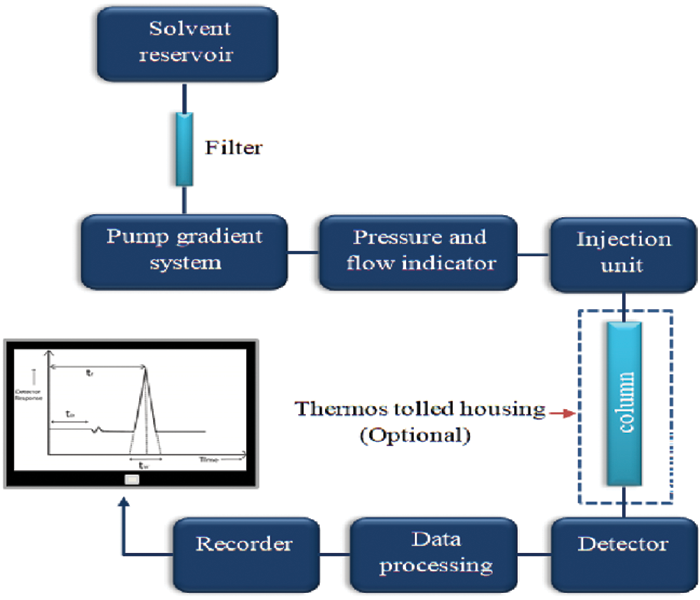
Components of a Chromatographic System
A typical chromatographic system consists of several key components that work in harmony to enable the separation, detection, and analysis of complex mixtures. Below is an overview of each component:
Mobile Phase
The mobile phase is the fluid that carries the analyte through the system. Depending on the technique, it can be a gas (in GC) or liquid (in LC).
.Composition must match analyte properties
.Affects retention time, resolution, and peak shape
.Requires high purity and degassing (in LC)
Stationary Phase
The stationary phase is the fixed medium in which separation occurs. It may be a solid (adsorbent), a liquid coated on a solid support, or a functionalized polymer.
.Determines selectivity and separation efficiency
.Available in various chemistries (C18, CN, NH2, ion-exchange resins, etc.)
.Often packed in columns, cartridges, or TLC plates
Column
The column houses the stationary phase and is the core of the separation process.
.Made of stainless steel, glass, or fused silica
.Comes in various lengths, diameters, and particle sizes
.Column performance is critical for resolution and peak shape
Injector (Sample Introduction System)
Introduces the sample into the mobile phase flow without disturbing the chromatographic process.
.Manual or automatic injection systems
.Loop or split/splitless injection (especially in GC)
.Important to ensure reproducibility and minimal band broadening
Detector
Detects the separated components as they exit the column and converts this information into an electronic signal.
.Types include UV/Vis, Fluorescence, Refractive Index, Conductivity, MS (Mass Spectrometry)
.Sensitivity, selectivity, and compatibility with analytes are crucial
.Calibration is essential for quantitative analysis
Data System / Software
Processes and visualizes the detector output to generate chromatograms, calculate retention times, peak areas, and perform quantitative analysis.
.Modern systems include Chromeleon, Empower, OpenLab, Clarity
.Supports method development, system suitability, validation, and audit trails
Pump (Liquid Chromatography Only)
Drives the mobile phase through the column at a controlled rate.
.Must deliver consistent flow rate and pressure
.Common types include isocratic, gradient, and binary/quaternary pumps
.Precision is essential for reliable retention times and reproducibility
|
Technique |
Stationary Phase |
Mobile Phase |
| Partition chromatography (e.g. LLE, HPLC) | Liquid on inert solid support | Liquid |
| Gas chromatography (GC) | Liquid on solid support (e.g. PEG) | Gas |
| Ion exchange chromatography | Ion exchange resin (solid) | Liquid |
| Size exclusion chromatography (SEC) | Porous polymer or gel beads | Liquid |
| Thin layer chromatography (TLC) | Silica gel or alumina layer on glass | Liquid |
| Paper chromatography | Cellulose paper | Liquid |